As molecular motors of the microtubule cytoskeleton, kinesin families play a vital role in intracellular transport. Like other ATPases, the activity of kinesins must be precisely regulated. Evidence showed that dysregulation of kinesin activity is linked to human neurodegenerative diseases. However, how cells instruct kinesins on when and where to function remains a mystery.
On April 24, 2025, Professor Guangshuo Ou from the School of Life Sciences at Tsinghua University published a research paper titled “Phosphorylation-dependent regional motility of the ciliary kinesin OSM-3” in the Journal of Cell Biology, revealing how NEK family kinases and the phosphatase PP2A regulate regional activity of the ciliary kinesin OSM-3. This study provides a new perspective on the spatial and temporal regulation mechanisms of kinesin activity.
Previous research from Guangshuo Ou’s lab found that ciliogenesis requires orchestrated collaboration between two kinds of kinesins, Kinesin-II and OSM-3. Kinesin-II powers intraflagellar transport (IFT) in the middle segment of the cilium, where OSM-3 remains inactive. Gradually, Kinesin-II hands over the IFT particle to the other ciliary kinesin, OSM-3, to complete the assembly of the distal segment. However, fluorescence lifetime imaging of this study suggested that OSM-3 had already released its autoinhibited conformation at the ciliary base, indicating an unknown mechanism keeps it inactive even in its extended conformation.
Through in vitro biochemical experiments and mass spectrometry, researchers revealed that NEKL-3 kinase can directly phosphorylate the motor domain of OSM-3 and suppress its ATPase activity. Single-molecule motility and microtubule gliding assays further confirmed that NEKL-3 significantly inhibits the molecular motility of OSM-3. Conversely, they found that overexpression of PP2A phosphatase at the transition zone or middle segment of cilium leads to premature activation of OSM-3, while loss-of-function mutation of PP2A reduces OSM-3 activity and shortens cilia.
These findings uncovered a phosphorylation-dependent mechanism that regulates the regional activation and inactivation of the ciliary kinesin OSM-3. Given conservation between the enzymes, this mechanism may also apply to the regional regulation of other kinesins. This study may offer new insights into the precise spatiotemporal regulation of molecular motor activity.
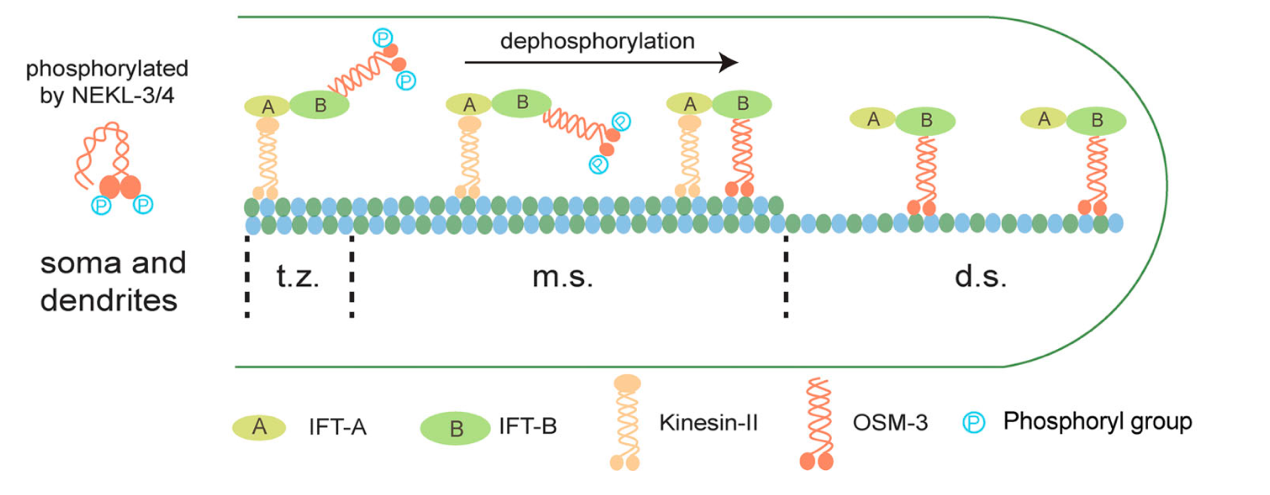
Figure 1. Diagram of proposed model on how phosphorylation regulates regional motility of ciliary kinesin OSM-3 (t.z.: transition zone; m.s.: middle segment; d.s.: distal segment).
This research was supported by the Cryo-Electron Microscopy Platform at Tsinghua University, as well as funding from the Beijing Frontier Research Center for Biological Structure, the Tsinghua-Peking Center for Life Sciences, the Ministry of Science and Technology of China, and the National Natural Science Foundation of China.
Article link: https://doi.org/10.1083/jcb.202407152
Editor: Li Han

